ao. Univ.-Prof. Mag. Dr. Eugen Libowitzky
eugen.libowitzky(a)univie.ac.at
Josef-Holaubek-Platz 2 (UZA II),
1090 Vienna
Room number: 2B375
T: +43-1-4277-53250

Curriculum vitae [pdf]
Fields of scientific interest
Hydrogen and hydrous species in minerals
Crystal chemistry of trace hydrogen and hydrous species in minerals
IR spectroscopy of hydroxides and hydrates, but also nominally anhydrous minerals
Phase transitions related to proton order-disorder
Short hydrogen bonds in minerals and synthetic inorganic materials
Dynamics of hydrogen in vacancies of crystal structures
General mineralogy
Reflected light investigations of ore minerals, study of ore deposits
Surface influence and anomalous effects in mineral optics
Determination of new minerals - and discreditation of old ones
Micro-analytics of minerals (SEM, EDX)
Previous and present work
REASONS FOR OPTICAL ANISOTROPY IN CUBIC ORE MINERALS
Since the beginning of this century ore microscopists were interested in the anomalous, optically anisotropic behavior of cubic ore minerals like pyrite, cuprite, and minerals of the spinel group. The unusual effect was attributed to the influence of tectonic stress, anomalous solid solutions series, incorporation of trace elements (As in pyrite, Zn in spinels), and influence of rough preparation methods.
During the study of approximately 500 ore mineral sections (ore microscopy, XRD, SEM) the following reasons for the anomalous anisotropy were revealed:
(1) The mineral donathite (discredited) is a spinel of the chromite-magnetite (ferrite) ss-series which exhibits tiny exsolution lamellae. Because the lamellae are sub-micron sized, because they are parallel to each other, and because they have two different sets of optical constants (one relating to magnetite, the other one to chromite), they show the effect of form birefringence. Etched samples reveal the sub-micron sized lamellae with a SEM.
(2) A similar phenomenon is found in extremely fine-zoned magnetites of skarn deposits. The parallel zones of sub-micron thickness and changing composition lead also to the phenomenon of form birefringence. Etching of the polished samples with HCl makes the fine zones also visible in the SEM.
(3) A careful examination of hundreds of pyrite, spinel, and cuprite sections which had been polished with diamond pastes, showed that they were optically anisotropic without exception. (The very strong anisotropy in cuprite had been used over decades as a determinative feature!) An investigation of the polished surfaces with the electron channeling pattern (ECP) technique on a SEM proved that the upper, polished surface layers were destroyed. The application of alkaline silica solutions in the final polishing process (a chemo-mechanical method) lead to optically isotropic sections and to undistorted surface layers in the ECPs. Examination of differently oriented polished sections revealed that the surface deformation by mechanical (diamond) polishing methods is related to the surface symmetry of the sections.
Related papers:
Libowitzky E (1991) Donathite: An intergrowth of magnetite and chromite, causing form birefringence. N Jb Miner Mh 10, 449-456
Libowitzky E (1993) Ursachen optischer Anisotropieeffekte in kubischen Erzmineralen. Berichte der DMG 1, 1993 (Add Vol Eur J Mineral 5), M-P-10,79, 246, abstract
Libowitzky E (1994) Anisotropic pyrite: A polishing effect. Phys Chem Minerals, 21, 97 - 103
Libowitzky E (1994) Optical anisotropy of cuprite caused by polishing. Can Mineral, 32, 353 - 358
Libowitzky E (1994) Optical anisotropy in the spinel group: A polishing effect. Eur J Mineral, 6, 187 -194
Libowitzky E (1994) Optical anisotropy of zoned magnetites due to form birefringence. Mineral Petrol, 52, 107 - 111
Burns PC, Hawthorne FC, Libowitzky E, Bordes N, Ewing RC (1997) Donathite discredited: A mixture of two spinels. N Jb Mineral Mh, 1997, 163-174
Libowitzky E (2001) The pseudo-biabsorption of trigonal rock-forming carbonates. N Jb Miner Mh, 2001, 67 - 79
HYDROUS SPECIES IN MINERALS
Hydrogen, as a major or trace constituent of minerals, has considerable influence on the physical properties of rocks and minerals (deformation behavior, rheology, conductivity). Hydrogen occurs usually in the form of OH groups or water molecules in minerals. If H containing minerals are stable to high temperatures and pressures, they may provide an important source of hydrogen in the Earth's interior. Hence, trace amounts of water in minerals like olivine, perovskite, pyroxenes, and garnets, as well as the behavior of the stoichiometric OH and water groups in the high-pressure mineral lawsonite are of interest. In addition, the features of hydrous species in oxide and silicate structures is important for technically applied materials like the microporous zeolites, precursors of ceramics, and proton conductors.
IR spectroscopy provides one of the most sensitive methods in determining hydrogen in minerals. Using polarized radiation and oriented crystal sections the orientation of O-H vectors in the structure can be determined, and even quantitative information can be obtained by accurate measurements and calibration procedures. Additional information on the behavior of OH and water molecules (if present in larger amounts) can be gained from other spectroscopic techniques (e.g. proton-NMR) and from X-ray and neutron diffraction.
(1) FTIR investigations of pure Mg-olivine (forsterite) revealed that trace amounts of hydrogen are incorporated in the form of OH defects which are related to Si vacancies. The polarized IR spectra show several sharp bands between 3500 and 3700 cm-1 which indicate different OH vector directions (predominantly II x) and additional trace elements in the coordination sphere of the OH groups.
(2) A similar mechanism was observed in CaTiO3 perovskite. The FTIR spectrum shows only two bands in the OH stretching region (around 3300 cm-1) which are indicative for OH incorporation in connection with a vacant Ca site. The stretching frequencies are in good agreement with a distorted environment around the vacancy. The type of OH defect is very similar to that of high-pressure MgSiO3 perovskite.
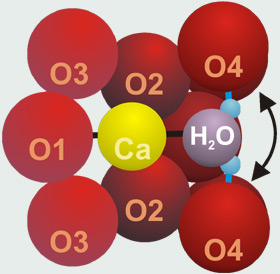
The room-temperature structure of lawsonite - The arrow indicates the hopping motion (dynamic disorder) of the water molecule
(3) Single-crystal X-ray diffraction of lawsonite,
The phase transitions were monitored by distinct X-ray reflections. They cause also nonlinearities in lattice parameters and optical constants.
An FTIR spectroscopic investigation of oriented lawsonite slabs resulted in a good correlation between band positions, H bond lengths, and OH vector directions at low temperatures. However, the smooth change of the spectra, the strong shift of absorption bands, and the unstable position of the water molecule between two proton acceptors, indicate rather a dynamic order-disorder transition than a displacive phase transition. These results are also confirmed by preliminary proton NMR and neutron diffraction studies.
The lawsonite-type mineral hennomartinite shows similar phase transitions at elevated temperatures. However, multiple disorder and twinning, as well as a monoclinic distortion at room-temperature (after a tempering process) draw a more complicated picture.
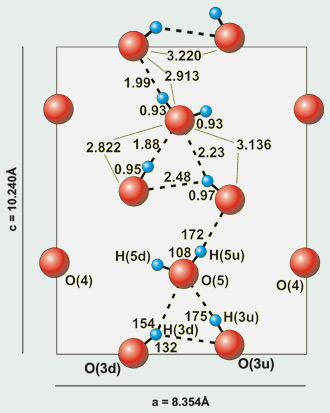
The water molecule and hydroxide groups in the channel of the hemimorphite structure
(4) Knowledge about lawsonite and about features of dynamic proton order-disorder and related phase transitions led also to the discovery of proton disorder and a phase transition at 98 K in hemimorphite,
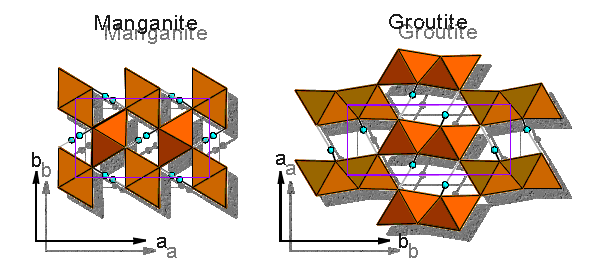
Structures of MnOOH: Manganite and groutite
(5) Polarized IR spectroscopic investigations of single-crystals of MeOOH (Me = Al, Fe, Mn) minerals showed similar spectra of all substances, the O-H stretching energies, however, are different and in good agreement with the respective hydrogen bond lengths. In addition, the in-plane and out-of-plane bending modes were observed. Peculiar absorption features in the spectra between the stretching mode and the bending modes were assigned to the anharmonicity-resonance enhanced overtones of the bending modes. Increasing anharmonicity leads to increased intensity of the mentioned band(s) and is related with decreasing hydrogen bond lengths.
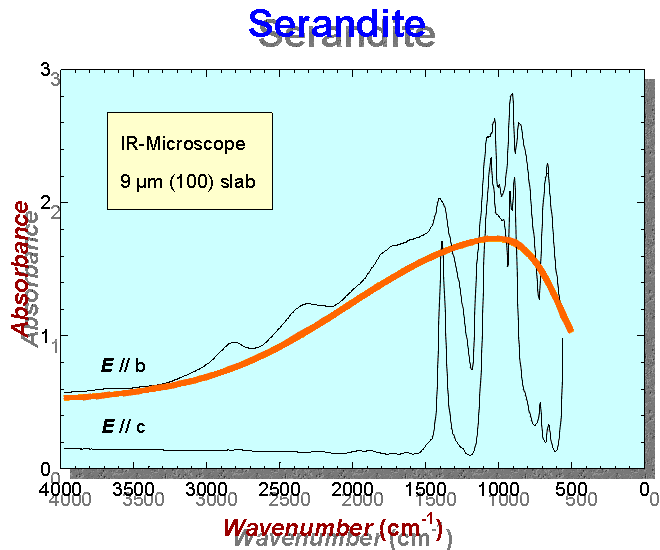
The broad OH absorption band in the IR spectrum of serandite
(6) Polarized infrared (IR) absorption spectra of oriented single-crystal slabs of mozartite
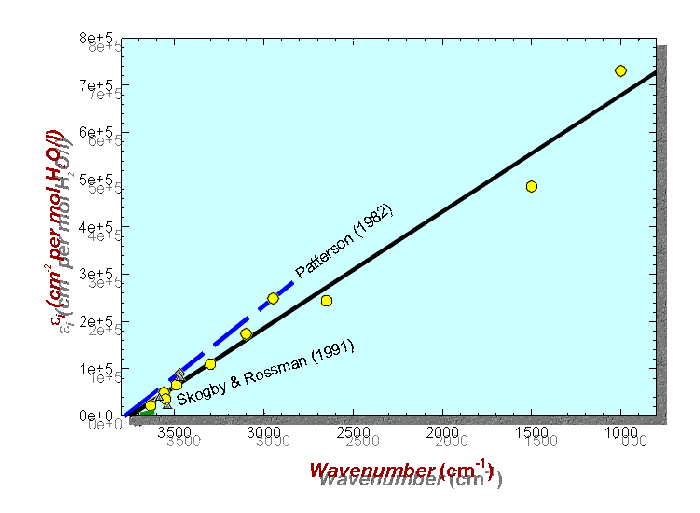
IR calibration line based on stoichiometric mineral hydrates and hydroxides
(7) IR spectroscopy is a useful tool also for the quantitative characterization of hydrous species in minerals. If proper experimental conditions are followed (polarized spectra of oriented or orthogonal crystal sections in cases of anisotropic crystals; knowledge about polarizer extinction ratios), the Beer-Lambert law (A = ·c ·t), which relates absorbance (A), concentration (c), and thickness (t) by the molar absorption coefficient (), may be used for the determination of water in geological materials. The molar absorption coefficient, however, can only be determined by calibration on standard materials with known water concentration. Experiments with different stoichiometric mineral hydrates and hydroxides confirmed that only the integrated absorbance Ai (band area) can be used among different materials. Further, a direct correlation between absorbance and water concentration fails, because the absorption coefficient is strongly dependent on hydrogen bond strengths. Hence, the resulting calibration line is expressed in relation to band energies () by:
(8) The frequency of an O-H stretching vibration is a valuable measure for the strength of a hydrogen bond. It is correlated to H bond lengths, i.e. O-H, O···O and H···O distances: The high-energy end is represented by very weak H bonds with d(O···O) 3.0 Å and at 3500 - 3700 cm-1, the low-energy end is found at very strong H bonds with d(O···O) even below 2.5 Å and bands at 700 - 1500 cm-1. Whereas previous d - correlation diagrams were limited either by insufficient number of data or by restricted data range, a recent d - correlation in minerals uses 125 d(O···O)- and 47 d(H···O)-data pairs from silicates, oxi-hydroxides, sulphates, etc. containing OH, H2O or H3O2 units with wavenumbers between 1000 and 3700 cm-1. A correlation function is established in the form = 3592 - 304·109 · exp(-d(O···O) / 0.1321), R2 = 0.96. Scatter of data is mainly caused by deviation from straight H bonds (avoided if d(H···O) data are used) and cationic effects.
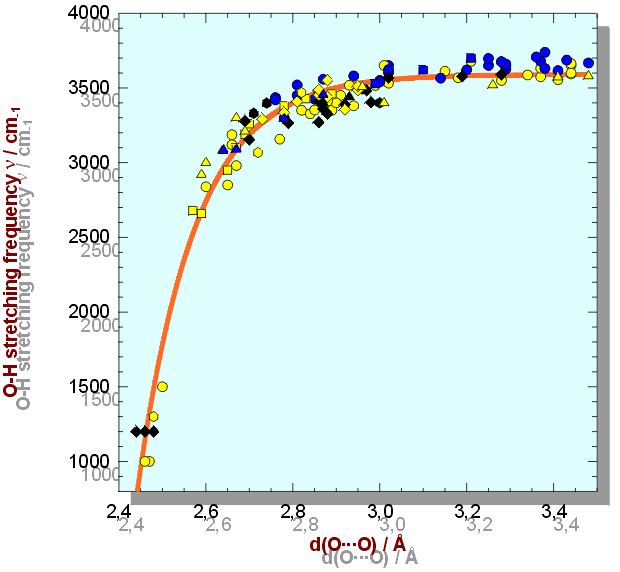
Correlation of O-H stretching frequency and d(O···O) in minerals
Related papers:
Armbruster T, Libowitzky E, Diamond L, Auernhammer M, Bauerhansl P, Hoffmann C, Irran E, Kurka A, Rosenstingl H (1994) Crystal chemistry and optics of bazzite from Furkabasistunnel (Switzerland). Mineral Petrol, 52, 113 - 126
Libowitzky E, Beran A (1994) OH-Defekte in Forsterit. Mitt Österr Miner Ges, 139, 331 - 333. Abstract.
Libowitzky E, Beran A (1995) OH-defects in forsterite. Phys Chem Minerals, 22, 387 - 392
Libowitzky E, Armbruster T (1995) Low-temperature phase transitions and the role of hydrogen bonds in lawsonite. Am Mineral, 80, 1277 - 1285
Libowitzky E, Armbruster A (1996) Lawsonite-type phase transitions in hennomartinite,
Beran A, Libowitzky E, Armbruster T (1996) A single-crystal infrared spectroscopic and X-ray investigation of an untwinned San Benito perovskite. Can Mineral, 34, 803 - 809
Libowitzky E, Rossman GR (1996) Principles of quantitative absorbance measurements in anisotropic crystals. Phys Chem Minerals, 23, 319 - 327
Libowitzky E, Rossman GR (1996) FTIR spectroscopy of lawsonite between 82 and 325 K. Am Mineral, 81, 1080 - 1091
Kozlova SG, Gabuda SP, Armbruster T, Libowitzky E (1996) Hydrogen atom localization in lawsonite using single-crystal PMR data. IUCR-1996 abstract
Libowitzky, E (1996) Order-disorder phase transitions in lawsonite and hemimorphite. Mitt ÖMG, 141, 132 - 133, abstract
Libowitzky, E (1996) Single-crystal IR spectroscopy of MeO(OH) minerals (Me = Al, Fe, Mn). Mitt ÖMG, 141, 134 - 135, abstract
Libowitzky E (1996) Proton disorder, phase transitions, and IR spectroscopy in minerals. EMSM, Kiev, abstract
Beran A, Libowitzky E (1996) OH-groups in natural perovskite - An IR spectroscopic study. Phase Transitions, 58, 211 - 215
Nyfeler D, Hoffmann C, Armbruster T, Kunz M, Libowitzky E (1996) Orthorhombic Jahn-Teller distortion and
Libowitzky E (1997) Wasserstoff-Unordnung und Phasenumwandlungen in Lawsonit und Hemimorphit. Habilitationsschrift, Universität Wien
Libowitzky E, Rossman GR (1997) IR spectroscopy of hemimorphite between 82 and 373 K and optical evidence for a low-temperature phase transition. Eur J Mineral, 9, 793-802
Libowitzky E, Kohler T, Armbruster T, Rossman GR (1997) Proton disorder in dehydrated hemimorphite - IR spectroscopy and X-ray structure refinement at low and ambient temperatures. Eur J Mineral, 9, 803-810
Kohler T, Armbruster T, Libowitzky E (1997) Hydrogen bonding and Jahn-Teller distortion in groutite,
Libowitzky E, Rossman GR (1997) Infrared spectroscopy: A quantitative approach to water in minerals. Ber DMG - Beih Eur J Mineral, 9, 222, abstract
Libowitzky E, Armbruster T, Beran A, Giester G, Hammer VMF, Hoffmann C, Kunz M, Nyfeler D, Rossman, GR (1997) Infrared spectroscopy of very strong hydrogen bonds in minerals. Ber DMG - Beih Eur J Mineral, 9, 221, abstract
Nyfeler D, Hoffmann C, Armbruster T, Kunz M, Libowitzky E (1997) Orthorhombic Jahn-Teller distortion and Si-OH in mozartite
Libowitzky E, Rossman GR (1997) An IR absorption calibration for water in minerals. Am Mineral, 82, 1111-1115
Libowitzky E, Beran A (1997) Hydrogen and hydrogen bonds in minerals. XIIth Conference "Horizons in Hydrogen Bond Research". Abstract
Beran A, Giester G, Libowitzky E (1997) The hydrogen bond system in natrochalcite-type compounds - An FTIR spectroscopic study of the H3O2− unit. Mineral Petrol, 61, 223-235
Hammer VMF, Libowitzky E, Rossman GR (1998) Single-crystal IR spectroscopy of very strong hydrogen bonds in pectolite,
Libowitzky E, Schultz AJ, Young DM (1998) The low-temperature structure and phase transition of hemimorphite,
Libowitzky E (1998) Wasserstoff und Wasserstoffbrücken in Mineralen. Mitt ÖMG, 143, 41 - 53
Armbruster T, Birrer J, Libowitzky E, Beran A (1998) Crystal chemistry of Ti-bearing andradites. Eur J Mineral, 10, 907 - 921
Libowitzky E (1998) OD-Charakter der Hemimorphitstruktur bei 20 K: Strukturuntersuchung mit TOF-Neutronen. Mitt ÖMG, 143, 332 - 333, abstract
Lager GA, Libowitzky E, Schultz AJ (1998) Neutron diffraction study of the low-temperature phase transitions in lawsonite. IMA98, A99, abstract
Libowitzky E (1999) Correlation of O-H stretching frequencies and O-H···O hydrogen bond lengths in minerals. Mh Chemie, 130, 1047 - 1059
Beran A, Libowitzky E (1999) IR spectroscopy and hydrogen bonding in minerals. In: Wright K, Catlow R (eds) (1999) Microscopic properties and processes in minerals. Kluwer Academic Publishers, Netherlands , 493 - 508
Libowitzky E (1999) Comparison of hydrogen bond distance - frequency correlations in solids. Ber. DMG - Beih Eur J Mineral, 11, abstract, in press
Hertweck B, Libowitzky E, Giester G (1999) Phase transitions in leonite-type compounds. Ber. DMG - Beih Eur J Mineral, 11, 104. Abstract
Sondergeld P, Schranz W, Kityk AV, Carpenter MA, Libowitzky E (2000) Ordering behaviour of the mineral lawsonite. Phase Transitions, Part B, 71, 189 - 203
Sondergeld P, Schranz W, Tröster A, Carpenter MA, Libowitzky E, Kitijk AV (2000) Optical, elastic, and dielectric studies of the phase transitions in lawsonite. Phys Rev B, 62, 6143 - 6147
Libowitzky E (2000) Phase transitions in minerals: Correlation of spectroscopic and diffraction data. 19th Eur Cryst Meeting Astracts, 137. Abstract
Libowitzky E, Giester G (2000) The crystal structure of soda at 110 K and 270 K. 19th Eur Cryst Meeting Astracts, 341. Abstract
Hertweck B, Armbruster T, Libowitzky E (2000) A single-crystal study of the low-temperature phase transitions in leonite-type compounds. 19 th Eur Cryst Meeting Astracts, 362. Abstract
Armbruster T, Kohler T, Libowitzky E, Friedrich A, Miletich R, Kunz M, Medenbach O, Gutzmer J (2001) Structure, compressibility, hydrogen bonding, and dehydration of the tetragonal Mn 3+ hydrogarnet, henritermierite. Am Mineral, 86, 147 - 158
Hertweck B, Libowitzky E (2001) IR and Raman spectroscopy of the phase transitions in leonite-type compounds. Bull Liaison SFMC, 13, 80. Abstract
Hertweck B, Libowitzky E, Schultz AJ (2001) Neutron diffraction study of the low-temperature phase transitions of Mn-leonite. Mitt Österr Miner Ges, 146, 109 - 110. Abstract
Hertweck B, Libowitzky E, Giester G (2001) The crystal structures of the low-temperature phases of leonite-type compounds, K2Me(SO4)2×4H2O (Me2+ = Mg, Mn, Fe). Am Mineral, 86, 1282 - 1292
Nasdala L, Beran A, Libowitzky E, Wolf D (2001) The incorporation of hydroxyl groups and molecular water in natural zircon (ZrSiO4), Am J Sci, 301, 831 - 857
Szalay V, Kovács L, Wöhlecke M, Libowitzky E (2002) Stretching potential and equilibrium length of the OH bond in solids. Chem Phys Lett, 354, 56 - 61
Hertweck B, Armbruster T, Libowitzky E (2002) Multiple phase transitions of leonite-type compounds: optical, calorimetric, and X-ray data. Mineral Petrol, 75, 245 - 259
Libowitzky E(2002) Hydrogen bonding in minerals and inorganic materials. SGK/SSCr Newsletter, 56 and 57, 7. Abstract
Hertweck B, Libowitzky E (2002) Vibrational spectroscopy of phase transitions in leonite-type minerals. Eur J Mineral, 14, 1009 - 1017
Beran A, Libowitzky E (2003) IR spectroscopic characterization of OH defects in mineral phases. Phase Trans, 76, 1 - 15
Halmer MM, Libowitzky E, Beran A (2003) IR spectroscopic determination of OH defects in spinel group minerals. Geophys Res Abstr, 5, 06742. Abstract
Libowitzky E , Giester G (2003) Washing soda (natron), Na2CO3×10H2O, revised: Crystal structures at low and ambient temperatures. Mineral Petrol, 77, 177 - 195
Giester G, Libowitzky E (2003) Crystal structures and Raman spectra of Cu(OH)F and Cu3(OH)2F4. Z Kristallogr, 218, 351 - 356
Libowitzky E (2003) Hydrogen and hydrogen bonds in minerals. Book of Abstracts, 46, LERM 2003, Nove Mesto (CZ). Abstract
Halmer MM, Beran A, Libowitzky E (2003) Detecting OH defects in iron-bearing ( IVFe2+) spinel phases by IR spectroscopy. Book of Abstracts, 27, LERM 2003, Nove Mesto (CZ). Abstract
Hertweck B, Libowitzky E, Schultz AJ(2003) The hydrogen bond system of Mn-leonite: neutron diffraction results in comparison with IR spectroscopic data. Z Kristallogr, 218, 403 - 412
Halmer MM, Libowitzky E, Beran A (2003) IR spektroskopische Untersuchungen von OH-Defekten in eisenhaltigen ( IVFe2+) Spinellphasen. Mitt Österr Miner Ges, 148, 156. Abstract
Libowitzky E, Beran A (2004) IR spectroscopic characterisation of hydrous species in minerals. In: Beran A, Libowitzky E (eds) (2004) "Spectroscopic Methods in Mineralogy", EMU Notes in Mineralogy 6, 227 - 279
Beran A, Libowitzky E (eds) (2004) "Spectroscopic Methods in Mineralogy", EMU Notes in Mineralogy 6, 661 pp
Mihajlovic T, Libowitzky E, Effenberger H (2004) Synthesis, crystal structure, infrared and Raman spectra of Sr 5(As 2O 7) 2(AsO 3OH). Mitt Österr Miner Ges 149, 67. Abstract
Bellatreccia F, Della Ventura G, Libowitzky E, Beran A, Ottolini L (2004) A calibration curve for the OH content in vesuvianite: a polarized single-crystal FTIR study. Mitt Österr Miner Ges 149, 14. Abstract
Bellatreccia F, Della Ventura G, Libowitzky E, Beran A (2004) The quantitative determination of B and H in vesuvianite: an FTIR spectroscopic study. 32 nd Int Geol Congress, Florence , G04.02. Abstract
Mihajlovic T, Libowitzky E, Effenberger H (2004) Synthesis, crystal structure, infrared and Raman spectra of Sr 5(As 2O 7) 2(AsO 3OH). J Solid State Chem, in press
Bellatreccia F, Della Ventura G, Ottolini L, Libowitzky E, Beran A (2004) The quantitative analysis of OH in vesuvianite: a polarized FTIR and SIMS study. Phys Chem Minerals, submitted
